Synthetic blood clot and blood analog
As clinical implementation of stent retrieval and aspiration thrombectomy increases, a need exists for physiologically relevant in vitro device efficacy testing. The development of standardized “soft” and “hard” synthetic blood clots that mimic the properties of human thrombi and are compatible with imaging technologies is critical to device testing. Synthetic clots allow researchers to extract information for assessing clot integration, modeling hemodynamic conditions, and quantifying thrombectomy physics. The blood analog allows for accurate modeling of physiological pressures, flows, and viscosity of human blood without the mess, shelf life, or biological hazard concerns.
Ingredients
Hydroxypropl methocellulose (HPMC) in solution
90% ethanol
DI water
- BDL-blood analog is a shear-thinning fluid that is – viscosity matched, non-toxic, easy to use, easy to clean, and a replacement for blood in benchtop flow models of the cardio/neurovascular system.
- Blood analog can be used with particle imaging velocimetry (PIV) to determine flow effects, and is compatible with fluoroscopic imaging for surgical simulations
- Made from nontoxic and stable components (Hydroxypropyl methocellulose (HPMC), DI water, and 90% ethanol)
- BDL synthetic clots are composed of polyacrylamide and alginate and are compatible with particle image velocity (PIV) and radiographic imaging techniques
- Clots have quantified mechanical properties comparable to “soft” and “hard” human clots.
- Literature defines human clot types based on composition, with soft clots containing ~60% red blood cells (RBCs) and hard clots (fibrin-rich) containing ~20% RBCs.
- These two relatively homogenous clot types represent 80% of the human clots removed via mechanical thrombectomy each year.
- Synthetic clots offer a platform for reproducible device testing. This provides a greater understanding of mechanical thrombectomy device efficacy, which may lead to quantifiable advances in device development and eventual improved clinical outcomes.
- Reproduceable “soft” and “hard” clots are formulated to be visible via PIV and angiography imaging.
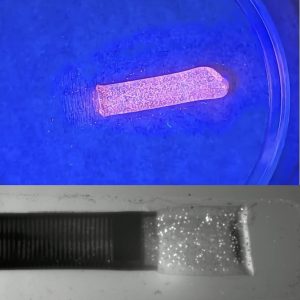
- The PIV synthetic clots contain fluorescent polymer microspheres for visualization with a UV light source during simulated surgical demonstrations and training.
- Discrete PIV clot ingestions can be captured in real time using a laser source to highlight the clot and a high-speed camera to capture the clot aspiration.
- PIV video analysis provides quantifiable flow velocities, stresses, strains, and force interactions between the catheter tip and the clot.
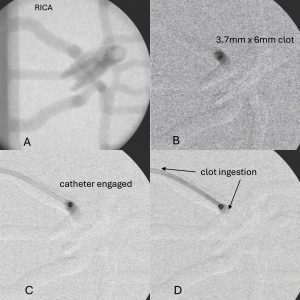
- The radiopaque synthetic clots contain barium-sulfate for visualization via angiography to provide clinically relevant in vitro visualization of the clot during full benchtop surgical simulations.
The synthetic clots developed here differ from commercially available clots (i.e. BioModex, animal, and human thrombi) as are supplied with documented and quantified mechanical properties for use with angiography or PIV imaging and won’t require special handling or biohazard cleanup. These clots are formulated to encompass the mechanical properties of ~80% of all clots removed during clinical procedures and can help clarify and quantify previously less-understood clot characteristics such as deformation, velocity, shear, and compressive forces that lead to optimal clot ingestion during endovascular device testing. Future research will investigate the creation of non-homogenous “specialty” clots and clots that can readily fragment and migrate distally.